A high standard for lowering blood pressure.
The Paradise™ Ultrasound Renal Denervation (RDN) System offers a high standard of therapy, harnessing the power of ultrasound energy to treat uncontrolled hypertension.
Hypertension remains a leading cause of cardiovascular issues, including heart failure, stroke, coronary heart disease, and more. And control rates around the globe are declining.
1.28B*
Adults globally
1.28B*
Adults globally
1 in 5*
Controlled
1 in 5*
Controlled
Take on uncontrolled or resistant hypertension with the Paradise™ Ultrasound Renal Denervation (uRDN) system. It is a minimally invasive procedure that lowers high blood pressure by treating overactive renal nerves with ultrasound energy.
With an exclusive design to ensure a safe and effective procedure, Paradise™ Ultrasound RDN offers a proven therapy option to lower blood pressure.1-3
*World Health Organization; https://www.who.int/news-room/fact-sheets/detail/hypertension; accessed on 7/09/2023
The Paradise™ System
Unleash the power of ultrasound renal denervation (uRDN)
Unleash the power of ultrasound renal denervation (uRDN)
Robust, durable results.
Deliver a lasting treatment for uncontrolled or resistant hypertension — proven safe and effective across several clinical trials.3,6-7
Off Medication
On Medication
Uncontrolled Hypertension
Resistant
Hypertension
–10.4mmHg
office systolic blood
pressure at 2 months ⁶
3/3
U.S. and Europe randomized
controlled trials ¹ ² ³
Zero
Major Adverse Events
at prespecified follow-up³
Made for efficiency.
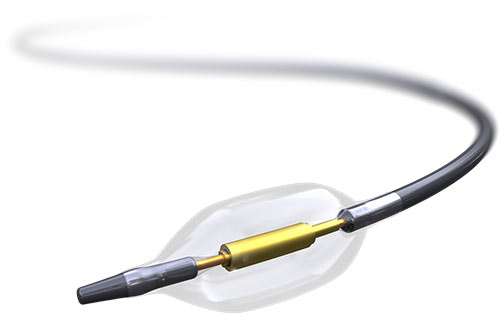
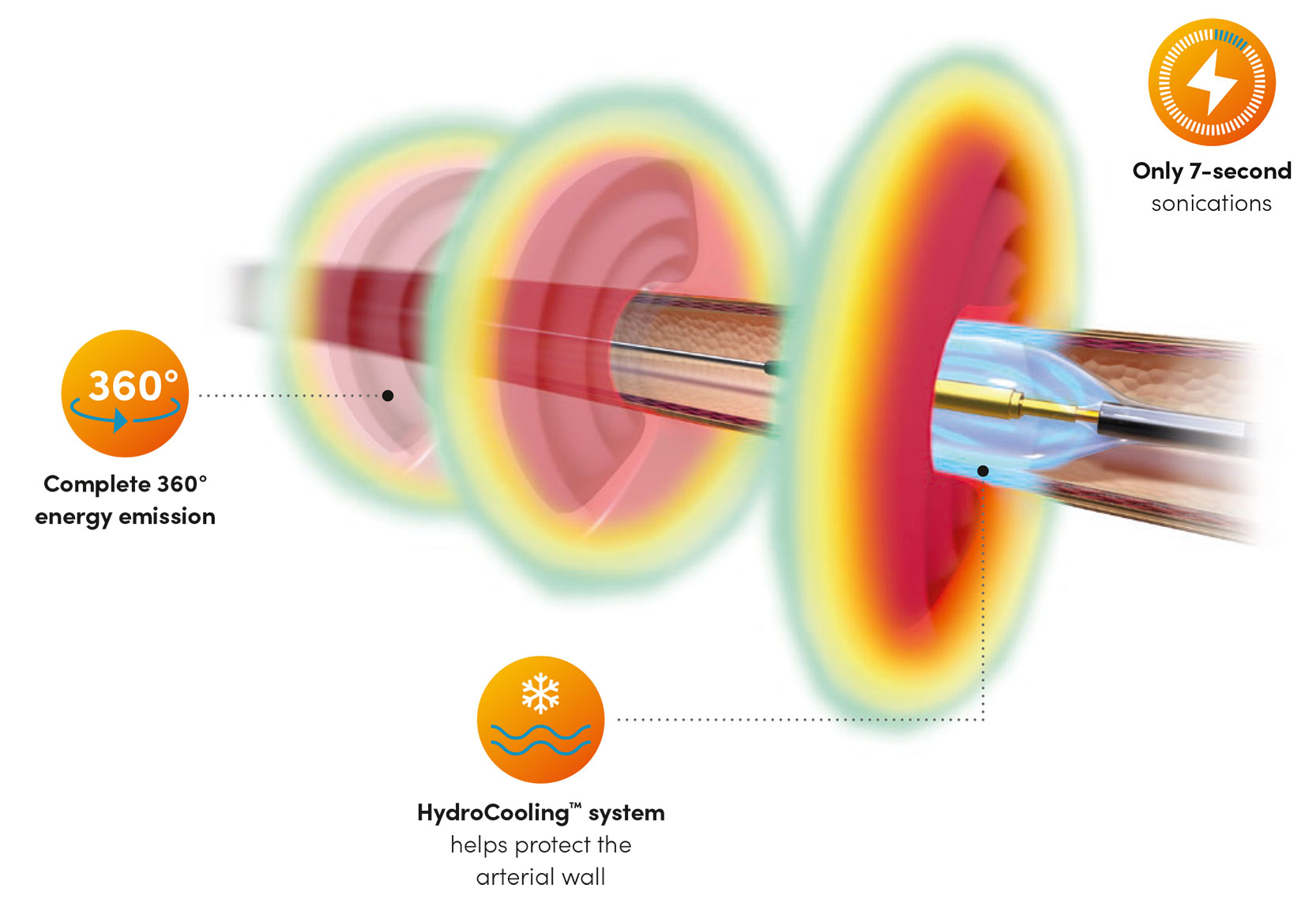
State-of-the-art Catheter4
- SonoWave 360 transducer
- Non-compliant balloon with HydroCooling system
- Atraumatic tip for patient safety
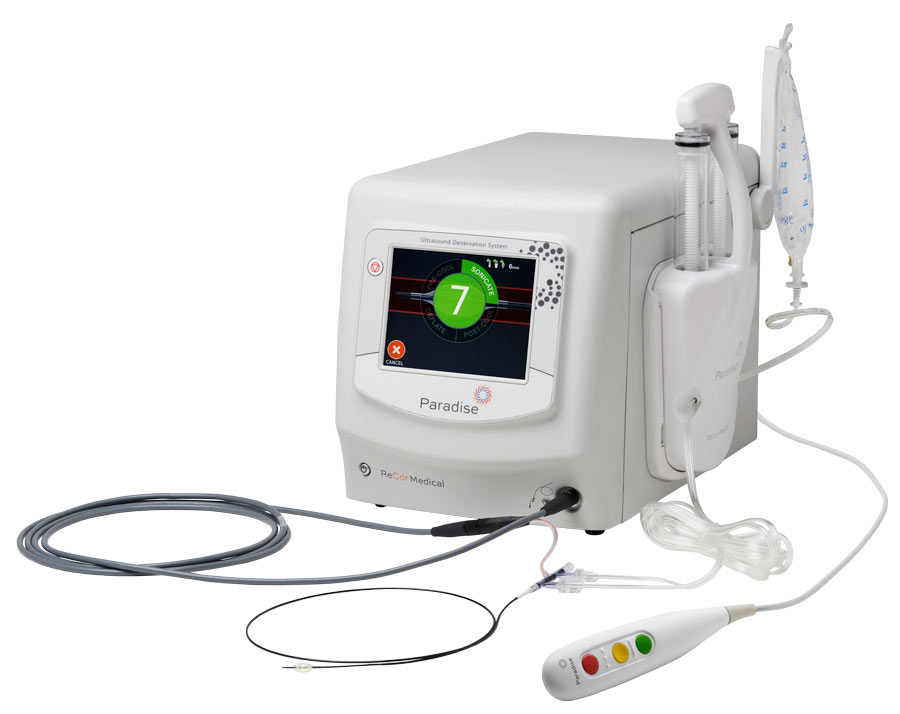
Intuitive Generator4
- Simple, user-friendly interface with ultra-efficient workflow
- Automatic energy level customized to the patient’s anatomy
- Hand-held remote to perform procedure from sterile field
References
- Azizi et al., Lancet. 2018;391(10137):2335-2345.
- Azizi et al., Lancet. 2021;397(10293):2476-2486.
- Azizi et al., JAMA. 2023;329(8):651-661.
- Data on file. Recor Medical. Inc.
- Pathak et al., EuroIntervention. 2015;11(4):477-84.
- Kirtane et al., JAMA Cardiol.2023; 8(5):464-473.
- Rader et al., EuroIntervention. 2022 ;18(8) :e677-e685.
- Zeijen et al., TCT 2023.
- Sakakura et al., JACC. 2014; Aug 19;64(7):635–643.
Indications for Use
The Paradise Catheter is indicated for percutaneous renal denervation.
Contraindications
The Paradise™ system is contraindicated for patients with any of the following:
Stented renal artery 2. Renal artery with stenosis of any origin >30% 4.Renal artery with Fibromuscular disease (FMD) 5.Renal artery with aneurysm 6. Iliac/femoral artery stenosis precluding insertion of the Paradise™ catheter 7.Pregnant 8. Less than 18 years of age 9.Known allergy to contrast medium
Warnings
- Failure to use the recommended balloon size may result in renal artery dissection, perforation, aneurysm, significant vasospasm requiring intervention, ablation of unintended tissues or structures, and/or no ablation of target tissue achieved.
- Energy emission in an unintended location may result in unintended tissue damage
- Do not move the Paradise™ Catheter during sonication.
- Do not sonicate in renal artery locations with visible plaque.
- Do not deliver sonications in an overlapping configuration.
Precautions
- Patients with known allergy to contrast medium should be appropriately treated.
- Only use specified coolant (Sterile water) for fluid supply. DO NOT USE SALINE.
- Avoid multiple inflations of the balloon to achieve apposition of the balloon to the renal artery wall; multiple balloon inflations may result in increased vessel trauma.
- The Paradise™ Catheter is for single use only. Do not resterilize or reuse. Reuse, reprocessing, or resterilization will compromise device integrity which may result in injury, illness, or death of the patient.
- Do not touch the Paradise™ Catheter balloon during sonication, as it may result in serious injury.
- The Paradise™ System may interfere with, or adversely affect the operation of cardiac pacemakers or other active implants, unless proper precautions have been taken or managed per the manufacturer’s instructions. When in doubt regarding possible hazards, seek qualified advice and/or consult with the manufacturer(s) prior to initiating a procedure. The Paradise™ Catheter is a Type CF, defibrillation-proof Applied Part.
Potential Adverse Effects of catheterization procedures
Allergic reaction to contrast, Arterio-enteric fistula, Arterio-venous fistula, Bleeding, Cardiopulmonary arrest, Complications related to pain and anti-anxiety medications, Death, Deep vein thrombosis, Edema, Embolism (pulmonary, renal, peripheral vasculature, plaque), Hematuria, Infection, Myocardial infarction, Pain, Vascular access site complications (pseudoaneurysm, pain, swelling, hematoma)
Risks of renal denervation procedure
Ablation or thermal injury to vessel, adjacent tissue or other structures, Acute kidney injury, Angina, Anxiety, Arrhythmia, Atrial tachycardia, Bradycardia, Gastrointestinal complications (diarrhea, nausea, vomiting), Hypotension/ Dizziness and/or Headaches, Hypertension, Hyperhidrosis, Pain (transient abdominal, lower back), Renal failure or renal insufficiency, Renal artery aneurysm or pseudoaneurysm, Renal infarction, Renal artery dissection, or perforation, Renal artery stenosis, Vasospasm, Vasovagal response, Stroke or transient ischemic event
Rx Only. Brief Summary – Prior to use, please reference the Instructions for Use