Research & Publications
Groundbreaking real-world data shows significant blood pressure reduction with Paradise™ uRDN System
December 10, 2025
Hypertension remains one of the leading preventable causes of cardiovascular disease worldwide, affecting millions of patients and contributing to severe health complications such as stroke, heart failure, and kidney disease. Despite the availability of lifestyle interventions and pharmacological treatments, a substantial proportion of patients continue to struggle with uncontrolled or resistant hypertension. For these individuals, innovative device-based therapies offer new hope. At the 2025 Transcatheter Cardiovascular Therapeutics (TCT) conference, Recor Medical presented compelling new data that reinforces the clinical value of its Paradise™ Ultrasound Renal Denervation (uRDN) system as a safe, effective, and durable solution for managing hypertension.
Real-World Data from the Global Paradise System (GPS) Registry
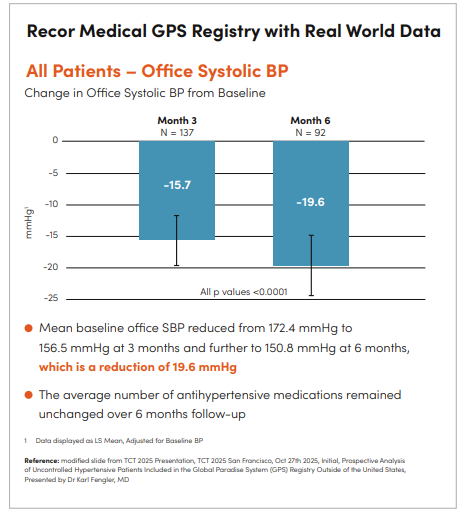
The Global Paradise System (GPS) Registry is an ongoing, real-world study designed to evaluate the long-term safety and effectiveness of the Paradise uRDN system across diverse patient populations. Conducted in nine countries outside the United States, the registry includes both prospective and retrospective cohorts, providing a comprehensive view of how the therapy performs in routine clinical practice.
The initial prospective analysis presented at TCT 2025 focused on 212 patients with uncontrolled hypertension, many of whom exhibited higher baseline blood pressures and risk profiles compared to participants in previous RADIANCE trials. Results were striking: at six months post-procedure, office systolic blood pressure (SBP) was reduced by 19.6 mmHg, while home SBP decreased by 14.4 mmHg (all p-values <0.0001). Importantly, these reductions were achieved without significant changes in the number of antihypertensive medications, highlighting the additive benefit of the Paradise uRDN system.
Beyond efficacy, procedural metrics demonstrated meaningful improvements compared to earlier studies. Procedure times, contrast volume, and fluoroscopy exposure were all reduced, reflecting growing operator experience and streamlined workflows. Safety outcomes were equally reassuring, with no major device-related concerns reported. Adverse event findings emulate those from trials and are consistent with what is expected in other endovascular procedures.
Sustained long term results
While short-term results are encouraging, long-term durability is critical for any hypertension therapy. To address this, Recor Medical presented a pooled analysis of its RADIANCE global clinical trial program, which includes three randomized, sham-controlled studies: RADIANCE-HTN SOLO, RADIANCE II, and RADIANCE-HTN TRIO. These trials collectively enrolled patients with mild-to-moderate and resistant hypertension, providing a broad evidence base.
The pooled data revealed that blood pressure reductions achieved with Paradise uRDN are sustained over time. At 24 months, office SBP remained 15.7 mmHg lower than baseline, confirming the therapy’s lasting impact. No new safety signals emerged during extended follow-up, further validating the system’s reliability. These findings are particularly significant given the progressive nature of hypertension and the challenges associated with long-term medication adherence.
Clinical and Practical Implications
The magnitude of blood pressure reduction observed in both the GPS Registry and RADIANCE trials is clinically meaningful. Studies have shown that even modest reductions in SBP can translate into substantial decreases in cardiovascular risk. For patients with uncontrolled or resistant hypertension, a 15–20 mmHg reduction could dramatically lower the likelihood of heart attack, stroke, and other complications.
Moreover, the consistency of results across controlled trials and real-world settings reinforces physician confidence in the therapy. The Paradise uRDN system’s ultrasound-based approach offers several advantages over alternative renal denervation technologies, including 360° circumferential energy delivery and the integrated HydroCooling™ system to protect the renal artery wall. These features contribute to procedural efficiency and safety, making the therapy an attractive option for interventionalists and patients alike.
Conclusion
Data presented at TCT 2025 mark a pivotal moment in hypertension care. With real-world evidence confirming significant BP reductions and long-term durability demonstrated in randomized trials, the Paradise™ uRDN system offers hope for millions living with uncontrolled hypertension.
Discover how the Paradise™ uRDN system is changing lives and shaping the future of cardiovascular health.
References: modified slide from TCT 2025 Presentation, TCT 2025 San Francisco, Oct 27th 2025, Initial, Prospective Analysis of Uncontrolled Hypertensive Patients Included in the Global Paradise System (GPS) Registry Outside of the United States, Presented by Dr Karl Fengler, MD
September 22, 2025
Despite advances in hypertension treatment, nearly 75% of U.S. adults still struggle with uncontrolled blood pressure. A new nationwide analysis highlights the vast potential – and risks – of renal denervation (RDN) as a therapeutic option.
June 26, 2025
Patients with uncontrolled high blood pressure saw meaningful reductions after switching to ultrasound renal denervation—even without increasing medications.
March 11, 2025
13 randomized sham-controlled trials (N=2,285) evaluated the efficacy and safety of ultrasound renal denervation (uRDN) and radiofrequency renal denervation (rRDN). uRDN demonstrated a greater reduction in ambulatory SBP across all time points.